
Space product assurance
Bioburden control of cleanrooms
Foreword
This Standard is one of the series of ECSS Standards intended to be applied together for the management, engineering and product assurance in space projects and applications. ECSS is a cooperative effort of the European Space Agency, national space agencies and European industry associations for the purpose of developing and maintaining common standards. Requirements in this Standard are defined in terms of what shall be accomplished, rather than in terms of how to organize and perform the necessary work. This allows existing organizational structures and methods to be applied where they are effective, and for the structures and methods to evolve as necessary without rewriting the standards.
This Standard has been prepared by the ECSS-Q-70-58 Working Group, reviewed by the ECSS Executive Secretariat and approved by the ECSS Technical Authority.
Disclaimer
ECSS does not provide any warranty whatsoever, whether expressed, implied, or statutory, including, but not limited to, any warranty of merchantability or fitness for a particular purpose or any warranty that the contents of the item are error-free. In no respect shall ECSS incur any liability for any damages, including, but not limited to, direct, indirect, special, or consequential damages arising out of, resulting from, or in any way connected to the use of this Standard, whether or not based upon warranty, business agreement, tort, or otherwise; whether or not injury was sustained by persons or property or otherwise; and whether or not loss was sustained from, or arose out of, the results of, the item, or any services that may be provided by ECSS.
Published by: ESA Requirements and Standards Division
ESTEC, P.O. Box 299,
2200 AG Noordwijk
The
Copyright: 2008 © by the European Space Agency for the members of ECSS
Change log
|
ECSS-Q-ST-70-58A
|
Never issued
|
|
ECSS-Q-ST-70-58B
|
Never issued
|
|
ECSS-Q-ST-70-58C
|
First issue
|
Introduction
The UN Outer Space Treaty of 1967 sets up the general principles applicable to the exploration and use of outer space. Article IX of the Outer Space Treaty constitutes the primary statement of international law: “States parties shall pursue studies of outer space, including the Moon and other celestial bodies, and conduct exploration of them so as to avoid their harmful contamination and also adverse changes in the environment of the Earth resulting from the introduction of extraterrestrial matter and, when necessary, adopt appropriate measures for this purpose”. Harmful contamination in that sense is defined as biological contamination, including organic-constituents, to protect the environment in order to allow future exobiology research. The Committee On Space Research (COSPAR) has established some planetary protection guidelines, based on the Outer Space Treaty. These guidelines impose requirements on spaceflight missions according to target body/mission type combinations.
The objective of this Standard is to ensure that the proper procedures to control the microbiological contamination in controlled environments are in place to meet the planetary protection constraints.
Scope
This standard establishes the principles and basic methodology for microbiological control of cleanrooms and associated controlled environments with planetary protection constraints.
This standard does not address:
the microbiological contamination control of spaceflight hardware;
molecular contamination control. Reference is made to other documents;
fire and safety regulations; for these, see regulatory requirements and other national or local documentation.
This standard does not lay down the methods for determining the microbiological and particulate cleanliness levels. Reference is made to other documents.
This standard may be tailored for the specific characteristic and constrains of a space project in conformance with ECSS-S-ST-00.
Normative references
The following normative documents contain provisions which, through reference in this text, constitute provisions of this ECSS Standard. For dated references, subsequent amendments to, or revision of any of these publications do not apply, However, parties to agreements based on this ECSS Standard are encouraged to investigate the possibility of applying the more recent editions of the normative documents indicated below. For undated references, the latest edition of the publication referred to applies.
|
ECSS-S-ST-00-01
|
ECSS system – Glossary of terms
|
|
ECSS-Q-ST-10-09
|
Space product assurance – Nonconformance control system
|
|
ECSS-Q-ST-20
|
Space product assurance – Quality assurance
|
|
ECSS-Q-ST-20-07
|
Space product assurance – Quality assurance for test centres
|
|
ISO 14644 part 1:1999
|
Cleanrooms and associated controlled environments - Part 1:Classification of air cleanliness
|
|
ISO 14644 part 2:2000
|
Cleanrooms and associated controlled environments - Part 2: Specifications for testing and monitoring to prove continued compliance with ISO 14644-1
|
|
ECSS-Q-ST-70-55
|
Space product assurance - Microbial Examination of Flight Hardware and Cleanrooms
|
Terms, definitions and abbreviated terms
Terms defined in other standards
For the purpose of this Standard, the terms and definitions from ECSS-S-ST-00-01 apply.
Terms specific to the present standard
action level
level set by the user in the context of controlled environment, and associated to specific requirements in the case that it is exceeded
alert level
level set by the user in the context of controlled environments, giving early warning of a drift from normal conditions, which, when exceeded, increased attention to the process is expected
aseptic
state of being free from all living microorganisms (i.e. free of bioburden)
In practice, it is usually described as a probability.
biobarrier(s)
barrier surrounding an item which prevents biological recontamination subsequent to microbial reduction procedures
bioburden
quantity of viable microorganisms measured with a specified assay
bioburden controlled
defined zone or facility in which bioburden is controlled by specified means
bioburden reduction
process or processes used to reduce the viable microbial population on an item to an acceptable limit
biocontamination
contamination of materials, devices, individuals, surfaces, liquids, gases or air with viable particles
biodiversity
identification of species of micro-organism, measured with specified assays
commissioning
planned and documented series of inspections, adjustments and tests carried out systematically to set the installation into the specified technical operation
controlled environment
defined zone in which contamination is controlled by specified means
disinfection
a process which destroys vegetative forms of microorganisms
Disinfection does not necessarily sterilize a surface or object.
formal system
system of biocontamination control with established and documented procedures
occupancy states
<as-built> condition where the installation is complete with all services connected and functioning, but with no production equipment, materials or personnel present
occupancy states
<at-rest> condition where the installation is complete with equipment installed and operating in a manner agreed upon by the customer and supplier, but with no personnel present
occupancy states
<operational> condition where the installation is functioning in the specified manner, with the specified number of personnel present and working in the manner agreed upon
planetary protection
policy and the technical implementations to prevent forward and backward contamination
sporicide
substance capable of destroying bacterial spores
sterile
state of being free from all living microorganisms (i.e. free of bioburden)
In practice, it is usually described as a probability.
sterilization
validated process used to render product free from viable micro-organisms
[ISO 11139]
Abbreviated terms
For the purpose of this Standard, the abbreviated terms from ECSS-S-ST-00-01 and the following apply:
|
Abbreviation
|
Meaning
|
|
AIV
|
assembly, integration, and verification
|
|
CFU
|
colony forming unit
|
|
COSPAR
|
Committee On Space Research
|
|
DHMR
|
dry heat microbial reduction
|
|
ESA
|
European Space Agency
|
|
ESD
|
electrostatic discharge
|
|
EGSE
|
electrical ground support equipment
|
|
FMECA
|
failure mode effects and critical analysis
|
|
GSE
|
ground support equipment
|
|
HEPA
|
high efficiency particulate air
|
|
HVAC
|
heating, ventilation, air conditioning, and cooling
|
|
IPA
|
isopropyl alcohol (isopropanol)
|
|
ISO
|
International Organization for Standardization
|
|
MGSE
|
mechanical ground support equipment
|
|
NASA
|
National Aeronautics and Space Administration
|
|
PP
|
planetary protection
|
|
WFI
|
water for injection
|
Principles
The activities related to requirements for bioburden control in cleanrooms, specifications, procedures and reports are described in Figure 41, and the related standardization requirements are captured in clause 5.
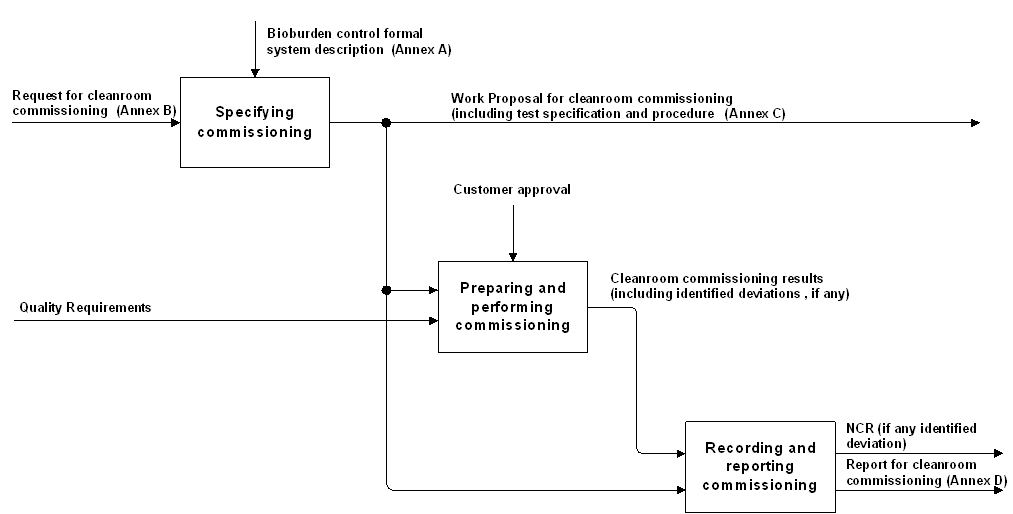 Figure 41: (Bioburden Control in Cleanrooms) examination process overview
Figure 41: (Bioburden Control in Cleanrooms) examination process overview
Clause 5.1 provides the requirements for bioburden control, clause 5.2 the operational requirements, and clause 5.3 provides the requirements for preparing, performing, recording and reporting cleanroom commissioning.
Additional information can be found in ISO 14698 Part 1 and Part 2.
Requirements
Bioburden control
Formal system
Establishment of a formal system
A formal system of bioburden control shall be established, formally approved, implemented, documented in conformance with Annex A, and maintained within bioburden controlled cleanrooms and associated environments.
The formal system assesses and controls factors that can affect the microbiological quality of the controlled environment.
Verification of the formal system
The result of bioburden monitoring as described in the formal system shall be examined with the periodicity established in conformance with Annex A.2.1, in order to verify that the formal system in use is functioning in conformance with the established procedures and the specified requirements have been fulfilled.
In some cases, the effective operation of the formal system cannot be appropriately verified without the establishment of supplementary tests and procedures, such as auditing, random sampling and analysis. This can also include the systematic verification of all working steps and equipment to ensure the system is functioning properly.
If verification indicates deviations from the established limits or a change in the microbiological status of the bioburden controlled environment, corrective actions as specified in the formal system per Annex A shall be initiated.
Action and alert levels
General
The user of the cleanroom shall set microbiological alert and action levels in accordance with the cleanroom cleanliness level and operations, and include them in the formal system specified in Annex A.
- 1 The following action levels can be used as a guideline:
- Action levels as per “Assay Procedure 2” in ECSS-Q-ST-70-55 for a bioburden controlled environment “during operations” are:
≤ 10 CFU/m3 for air samples;
≤ 2000 CFU/m2 for surfaces;
≤ 1 CFU/glove print (5 fingers).
- Action levels as per “Assay Procedure 2” in ECSS-Q-ST-70-55 “during aseptic operations” are:
< 1 CFU/m3 for air samples
< 400 CFU/m2 for surfaces;
< 1 CFU/glove print (5 fingers).
- 2 Aseptic operations cannot be ensured without laminar air flow. This can cause undesirable electrostatic charging effects and needs to be managed properly.
Alert levels shall be compatible with the action levels and the facility commissioning phase.
The use of statistical methods and trend analysis shall be applied to establish alert and action levels and for routine monitoring.
See Annex E.1 for commissioning phase and Annex E.2 for guidelines on alert and action level.
The appropriate representative responsible for control of bioburden controlled environments shall be notified in case of alert and action level events.
Investigation of alert and action level events
In the case of an alert level event, the following shall be performed:
- Review of record of laboratory tests and deviations, equipment performance, training of personnel, trend analysis, and any possible contributory anomalies or discrete events.
- A deviation report.
- 1 Additional information on the state of the environment (e.g. particulate level) should be evaluated.
- 2 Alert level events can be associated to corrective action, or not, depending on whether the cause is under control or not.
In the case of an action level event, an investigation shall be performed, which includes: - Root cause analysis.
- Corrective action.
- Impact on hardware.
- A deviation report.
See Annex E.2 for guidelines on elements to be covered with the investigation.
Three consecutive alert level events shall be treated as action level event.
Operational requirements
Cleanroom class
Bioburden controlled environments shall be equivalent at least to airborne particulate cleanliness class ISO 7 “in operation” in conformance with ISO 14644- Part 1.
- 1 This airborne particulate cleanliness class is applicable to cleanrooms or clean zones within cleanrooms.
- 2 Achieving the airborne particulate cleanliness class is not necessarily a matter of changes in the cleanroom or clean zone design or HVAC system but can potentially be achieved by applying proper access control, gowning, and procedures.
Bioburden controlled environments shall be continuously monitored in conformance with ISO 14644- Part 2. - 1 In some cases, aseptic operations can only be ensured if a more stringent particulate control (e.g. ISO 5) is applied. Normally such conditions are provided by laminar air flow environments with a controlled environment in the background.
- 2 For commissioning of bioburden controlled environment, see Annex E.1.
Applicability of bioburden control
Bioburden control shall apply to all items entering a bioburden controlled environment.
- 1 This includes e.g. any (compressed) gases, liquids, equipment, and garment.
- 2 Bioburden control is either via cleaning/sterilization and assays and/or isolation with biobarriers.
- 3 Bioburden control for the regular air supply of the cleanroom is through HEPA filters.
Restrictions
Equipment
All equipment used for bioburden control (including HVAC system) shall be subject to validation and planned maintenance.
The return to use of all equipment used for bioburden control shall be subject to approval of appropriate representatives responsible for the bioburden control of the environment and/or formal revalidation of the environment.
The layout of equipment shall provide for ergonomics that optimize comfort and movement of operators.
Material flow shall be optimized to prevent unnecessary activities that could increase the potential for introducing contaminants.
The design of equipment used in bioburden controlled environments should limit the number and complexity of bioburden controlled interventions by personnel.
Equipment design shall be appropriate to facilitate ease of sterilization.
The effect of equipment design on the cleanroom environment shall be addressed.
Horizontal surfaces or ledges that accumulate particles shall be avoided to the extent practicable.
Equipment shall not obstruct airflow and, in critical areas, its design shall not disturb unidirectional airflow.
Cleaning solutions
Cleaning solutions used shall be sterile prior to use.
See Annex E.5.15.8 for guidelines on cleaning solutions.
A cleanroom cleaning program shall be implemented in each controlled environment.
Personnel movement
The number of personnel in a bioburden controlled environment shall be minimized.
The flow of personnel shall be designed to limit the frequency with which entries and exists are made to and from a bioburden controlled environment.
To prevent changes in air currents that introduce lower quality air, personnel movement adjacent to the critical area shall be restricted.
Preparatory activities (e.g. adhesive mixing) shall be conducted in dedicated external or peripheral areas, with due regard to requirements for spacecraft microbial contamination requirements.
Bioburden monitoring of cleanroom environment
The biodiversity in bioburden controlled environments shall be characterized at the commissioning phase.
The bioburden shall be monitored with active air sampling and active surface sampling devices.
For routine testing of airborne bioburden either impaction/impingement sampler or filtration sampler shall be used.
As per procedure in ECSS-Q-ST-70-55.
For routine testing of airborne bioburden the total sample volume shall not be less than 1 m3.
For routing testing of surface bioburden either swabs, wipes, or contact plates shall be used.
As per procedure in ECSS-Q-ST-70-55.
For routine testing of surface bioburden the total sample area for swabs and contact plates shall be on the order of 0,0025 m2 (25 cm2).
For routine testing of surface bioburden the total sample area for wipes shall be between 2/3 m2 and 1 m2 maximum.
All bioburden and biodiversity assays shall be according to ECSS-Q-ST-70-55.
Sampling plan
General
A sampling plan shall be developed through the formal system (i.e., in the commissioning phase), and documented in conformance with Annex A.
Frequency of sampling
The frequencies of sampling shall be developed using the selected formal system.
This system shall encompass:
- initial monitoring (i.e. during commissioning phase),
- routine monitoring, and
- contingencies for contamination events.
Sampling sites
Sampling sites shall be determined through the selected formal system, and included in the sampling plan.
The sampling locations are established based on the risk assessment performed leading to the identification of risk zones and operational steps at risk.
The location of the designated sampling sites shall be selected in order to ensure all potential contamination sources are monitored.
- 1 That means, in addition to the “risk zone”, the designation and identification in the plan of areas such as access points, storage areas with significant levels of material or hardware and significant impedance to air flow, or areas of heavy personnel traffic.
- 2 More than one sample can be taken at each site and different numbers of samples can be taken at different locations.
Sampling shall be carried out at the bioburden control points defined in a written procedure which identifies the locations and frequency of sampling under nominal and non-nominal scenarios.
A schematic representation (map) of the facility showing the samples locations is considered to be essential.
Identification of samples
The labelling of each sample shall carry the information or coding to provide traceability of the sample.
The labelling of each sample shall include the following information as a minimum:
- Collection site (facility).
- Sampling location within facility.
- Occupancy state at time of sampling.
- No. of personnel present at time of sampling.
- Date and time of collection.
- Name of person collecting the sample.
- Size of sample in units of m3 (volume) or cm2 (surface area).
- Details of all activities being performed at the time of sampling.
- Sampling method(s), in conformance with ECSS-Q-ST-70-55.
- Analysis method(s), in conformance with ECSS-Q-ST-70-55.
- Any deviations from the requirements of the sampling plan.
Processing of samples
The procedures in conformance with ECSS-Q-ST-70-55 shall be followed.
Expression, interpretation and reporting of results
Quantitative results shall be expressed as colony-forming-units (CFU) per m3 (volume) or m2 (surface area).
To assist in interpretation, results shall be reviewed over extended periods to determine trends. Bioburden and biodiversity trend analysis shall be provided.
Training
A training programme shall be established in due time to allow appropriate training of all personnel.
See Annex E.3 for guidelines on the training programme.
Cleaning personnel shall be dedicated people.
‘Regular’ cleaning personnel from the site cleaning service provider are not necessarily appropriate.
Personnel
All personnel shall undergo regular medical screening to establish that they do not have a medical condition that can compromise the integrity of the bioburden controlled environment.
See Annex E.5.11 for examples of medical conditions of concern.
Hygiene programmes should be established and should include procedures relating to the health, hygiene practices and clothing of personnel.
Only personnel with the appropriate level of training and medical clearance shall access the bioburden controlled environments, including. personnel for cleaning and maintenance.
Non-essential personnel shall not be permitted in the bioburden controlled environment.
For accessing and working in a bioburden controlled environment, personnel shall wear at least a coverall, hood, face mask, boots, and two layers of sterile gloves.
A strict personnel monitoring program shall be established.
See Annex E.5.11 for guidelines
Cleanroom garments
Garments shall be compatible with ISO 7 cleanroom standards, or better.
The particulate filtration efficiency of the garment material should be at least equivalent to the class of cleanroom employed.
Sterile gloves shall be compatible with repeated alcohol washes (IPA 70% or ethanol 70%) washes.
The glove material should not contaminate the solvents by leaching, or degrade due to contact.
Cleanroom commissioning
The customer shall request a cleanroom commissioning in conformance with Annex B.
For safety and security, the test centre shall be in conformance with ECSS-Q-ST-20-07, clause 9.
Example of safety requirements are hazard and health requirements. Example of security requirements are access control requirements.
The supplier shall respond to the request specified in requirement 5.3a with a cleanroom commissioning proposal in conformance with Annex C for customer approval.
The supplier shall run the approved cleanroom commissioning campaign as described in Annex C.
An example of an approved cleanroom commissioning approach is given in Annex E.
The supplier shall submit the Cleanroom commissioning report in conformance with Annex D to the customer for approval
ANNEX(normative)Bioburden control formal system description - DRD
DRD identification
Requirement identification and source document
This DRD is called from ECSS-Q-ST-70-58, requirement 5.1.1.1a and 5.2.5.1a.
Purpose and objective
The bioburden control formal system is established to assess and control factors that can affect the microbiological quality of the process.
Expected response
Scope and content
The bioburden control formal system description shall include:
- Limits established to ensure control, including alert and action level as established in conformance with requirement 5.1.2.1a.
- Description of the monitoring scheme and schedule established.
- Description of the validation procedures established in conformance with clause 5.1.1.2 to verify that the formal system is working properly.
- Corrective actions established in conformance with requirement 5.1.2.2b.2 to be taken when a monitoring result indicates that a particular factor of the process is not under control.
- Risk assessment for bioburden control.
For example: Process FMECA.
- Description of the training procedures and access constraints established in conformance with clause 5.2.6.
- Documentation to be established and maintained.
- Process implementation documentation.
For example: Logbooks.
- Periodic reviews/audits to be performed.
- Maintenance status of equipment.
- Cleanroom cleaning programme.
- A training programme.
- Description of the sampling plan, including at least:
- The cleanliness level of the cleanroom to be applied and the appropriate degree of bioburden control for the activity being conducted.
- The sampling location(s) identified in conformance with clause 5.2.5.3, including the location of the primary “risk zone”; i.e.; the work area immediately adjacent to any contamination-critical hardware and the work activities/personnel associated with this location.
- The minimum number of samples to be taken in order to ensure representative results.
This number is dependant on the sample size/ volume. For small sample sizes, representativeness of the results cannot be guaranteed if this number is not significantly increased.
* Frequency of initial monitoring sampling, established in conformance with requirement 5.2.5.2b.1.
* Frequency of normal routine sampling, established in conformance with requirement 5.2.5.2b.2.
* Frequency of sampling for specific cases, established in conformance with requirement 5.2.5.2b.3, including at least the following cases:
Suspected contamination events.
Action levels are exceeded.
Alert levels are exceeded (consecutively).
A change in the biodiversity.
After prolonged shut-down of activities.
After any significant maintenance work has been undertaken on the cleanroom.
- 1 For example: Maintenance on ventilation system, changes to furnishings or fittings, introduction of significant quantities of new hardware or equipment.
After changes to the process that affect the cleanroom environment.
After recording of unusual results.
After non-routine changes to the cleaning or disinfection procedures.
After unplanned incidents that could contribute to contamination.
After any incidence of non-conformance to cleanroom operational specifications.
- 2 For example: Breaches of personnel dress/conduct code; out of specification airborne particulate levels, out of specification temperature or humidity levels, exceeding maximum no. of personnel, or introduction of non-sterile items or hardware.
Sampling method(s), established in conformance with clause 5.2.5.5.
Analysis method(s), established in conformance with clause 5.2.5.6.
Factors pertinent to a particular situation that can affect results.
Ambient temperature, lead-time to processing of samples.
The impact of operations, personnel and equipment, which contribute to contamination.
Special remarks
None.
ANNEX(normative)Request for cleanroom commissioning - DRD
DRD identification
Requirement identification and source document
This DRD is called from ECSS-Q-ST-70-58, requirement 5.3a.
Purpose and objective
The purpose of this DRD is to specify the content of a request for cleanroom commissioning.
Expected response
Scope and content
The request for cleanroom commissioning shall include or refer to the following information:
- reference in making ECSS-Q-ST-20 and ECSS-Q-ST-10-09 applicable,
- objective of the cleanroom commissioning,
- background and justification to the cleanroom commissioning,
- cleanroom to be commissioned,
- description of cleanroom commissioning activity, and
- deliverables.
Special remarks
None.
ANNEX(normative)Cleanroom commissioning specifications and procedures (Work Proposal) - DRD
DRD identification
Requirement identification and source document
This DRD is called from ECSS-Q-ST-70-58, requirement 5.3c.
Purpose and objective
The work proposal is a document that defines the test activity for cleanroom commissioning. The work proposal for cleanroom commissioning is prepared by the test house, which is responsible for the test activity, and it is submitted to the customer for review and approval.
Expected response
Scope and content
The WP shall include or refer to the following information:
- A proposed work description giving:
- the objectives of the cleanroom commissioning activity,
- cleanroom facilities to be commissioned,
- test facilities, test procedures and reference to standards,
- test conditions (i.e. environment, properties evaluated and measurement techniques),
- expected test output.
- A proposed settlement describing the test procedures and any deviation from the conditions initially requested by the customer.
- A financial and administrative proposal including:
- responsible person for the activity,
- list of deliverable items,
- work breakdown structure defining the required operations (i.e., preparation of specimens, testing, evaluation of results, reporting) and responsibilities,
- time schedule,
- travel and subsistence plan (if applicable),
- itemized cost list, and
- milestone payment plan.
Special remarks
None.
ANNEX(normative)Cleanroom commissioning report - DRD
DRD identification
Requirement identification and source document
This DRD is called from ECSS-Q-ST-70-53, requirement 5.3e.
Purpose and objective
The purpose of the cleanroom commissioning report is to provide quantitative evidence that the items were tested according to the sterilization compatibility test specifications and procedures.
Expected response
Scope and content
The cleanroom commissioning report shall include or refer to the following information:
- Description of the purpose, objective, content and the reason prompting its preparation.
- Description of the cleanroom to be commissioned or a reference to the document containing its identification characteristics.
For example: Request for cleanroom commissioning.
- Description of the tools, methods, and procedures for cleanroom commissioning.
It often consist in describing the as- run test procedure as well as any deviation from the initial test procedure (including a discussion of possible effect on test).
- The cleanroom commissioning results.
- Discussion about the test results.
- Conclusion and recommendations.
Special remarks
None.
ANNEX(informative)Cleanroom operation
Commissioning activities
Elements
The commissioning phase of a bioburden controlled environment should contain the following elements:
One detailed bioburden and biodiversity evaluation at rest.
Five detailed bioburden and biodiversity evaluation at (simulated) operation.
Establishing trends on bioburden and biodiversity.
Establishing alert and action levels.
Establishing of monitoring scheme.
- 1 The commissioning phase can take several weeks to months with the five detailed bioburden and biodiversity evaluation done in intervals during this period.
- 2 The biodiversity evaluation should go to the species level.
- 3 Spare MGSE/EGSE should be used for the commissioning phase. In case equipment is not available for the commissioning phase, a delta-commissioning should be performed.
- 4 The commissioning phase should be used for the training of personnel.
- 5 The commissioning phase should be used to establish routine bioburden monitoring schedules and locations for each different cleanroom activity type/activity level.
Action and alert level events
Investigation
An investigation after an action or alert level excursion should be initiated immediately and contain the following elements:
Clearly identified reason for the investigation.
Description of environment.
Type of contaminant.
Review sampling personnel (interviews).
Review sampling techniques.
Review analysis techniques.
Review cleaning, disinfection and sterilization records.
Review personnel monitoring, gloves and gown results.
Review manufacturing records of items affected.
Review overall environmental control and monitoring data of the environment, including associated environments (e.g., access areas).
Review type of operations carried out.
Review environment cleaning and sanitation records, including from associated environments (e.g., access areas).
Review air pressure across the filters and inspect for any leaks in the filter.
Check differential pressure.
Evaluate mechanical equipment in the environment as source of contamination.
Sampling error
If sampling/analysis failure is identified as cause for the excursion, re-test results substitutes the original (faulty) test result. The original (faulty) test result should be retained for the records, with the appropriate explanation. If no sampling/analysis error is identified in the original test, the original test results cannot be invalidated and a detailed investigation should be performed.
Inconclusive findings
For inconclusive investigations where the cause of the excursion is not revealed, the out-of-specification results should be retained in the record together with the re-test analysis and should state the disposition of the item under investigation.
Microorganism Identity
Organism identity should be included as alert and action criteria. Atypical organisms even at numbers below the prescribed levels, should be included as triggers for action.
The term “atypical organisms” describes microbes that have not previously been identified during the biodiversity evaluation as part of the commissioning phase.
Frequency
Alert and action levels for new facilities (i.e., during commissioning) should be revisited every three to four months. For established facilities with continuous operation (i.e., after commissioning), an annual review is sufficient.
Training programme
Overview
Training cannot be appropriately implemented without the development of useable procedures and training materials, coupled with documentation and record keeping of the performance elements applied, as well as a system for verification of training. Training can be conducted with the organization, or outside by an independent organization. The training should be conducted before an individual is permitted to enter the controlled area.
Training levels
Different levels of training should apply depending on the individual task descriptions. The following levels should be used:
Level 1: Highest level of training; personnel can work without direct supervision in bioburden controlled environment on flight hardware (e.g., supervisor, engineer, quality assurance engineer, hardware owner, etc.).
Level 2: Personnel can only work with direct supervision in bioburden controlled environment on flight hardware (e.g. PP support staff, general support staff, temporary visitors).
Level 3: Personnel can only work with indirect supervision (e.g., video surveillance) in bioburden controlled environment in the vicinity of protected flight hardware (e.g. maintenance and cleaning personnel).
Sampling and analysis
The personnel in charge of the performance of the sampling and analysis methods should be trained in the following aspects of biocontamination control:
Basic principles of microbiology.
Fundamental of applied microbiology, hygiene and epidemiology.
Good employee aseptic techniques and precautions.
Principles of environmental control process.
Microbiological sampling techniques.
Basic principles of microbiological hazard analysis.
Understanding of microbiological alert and action levels.
Principle of trend analysis.
Specialized training on all required laboratory method, and on any automated system to be used to assist microbial identification.
Training verification
Training verification procedures should be established.
Training content
The training programme should contain the following elements:
General policy and requirements.
Microbiology.
Hygiene.
A specific section on how to operate in a bioburden controlled environment.
- 1 The latter should be specific to the training level and dominated by practical exercises in a representative environment, including work flow and process control, gowning, and cleanroom discipline.
- 2 Refresher training course should be provided on a regular basis.
General guidelines for cleanroom design and operation
Design; general
Access to the bioburden controlled environment should be through successive layers of controlled environments (see Figure E-1), e.g., from uncontrolled environment through a standard ISO 8 environment, to a standard ISO 7 environment before entering the bioburden controlled ISO 7, environment. Different environments can be accommodated in a single cleanroom.
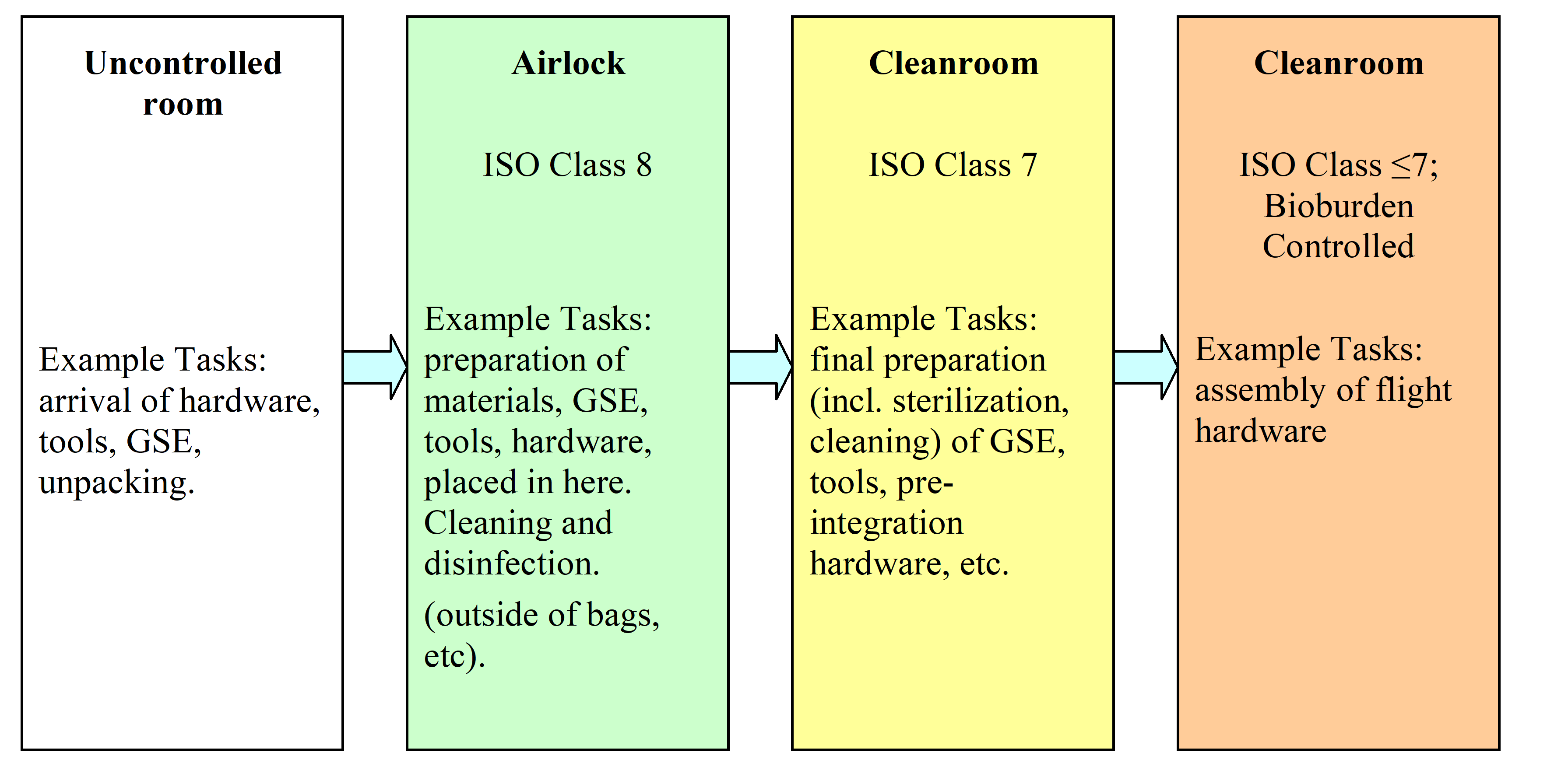 Figure: Cleanroom environment schematic
Figure: Cleanroom environment schematic
Airflow
Airflow patterns should be evaluated for turbulence or eddy currents that can act as a channel or reservoir for air contaminants in the aseptic area. In situ air flow analysis in the should be performed in the critical area to demonstrate the maintenance of the unidirectional air flow and the sweeping action over and away from the sterile equipment under dynamic conditions.
Videotape or other recording mechanisms have been found to be useful aides in assessing airflow initially as well as facilitating evaluation of subsequent equipment configuration changes.
Access
Hardware and personnel
Separate entry pathways for hardware (including e.g. , and flight hardware) and personnel should be provided.
Door operations
Automatic door operations should be utilized to reduce cross contamination and to facilitate the controlled air movement between adjacent cleanrooms with different cleanliness class.
Layout design
Surfaces
All exposed surfaces in the bioburden controlled environment should be smooth, impervious and unbroken. There should be no uncleanable surfaces and recesses. False ceilings should be sealed to prevent contamination from the space above them.
Sliding doors should not be used for this reason.
Pipes and ducts
Pipes and ducts and other utilities should be installed so that they do not create recesses, unsealed openings and surfaces which are difficult to clean/disinfect.
Changing rooms
Changing rooms should be designed as airlocks and used to provide a physical separation of the different stages of changing. The final stage of the changing room should be at the same grade as the areas into which it leads.
Hand washing and disinfection
In changing room
Hand washing facilities should be provided only in the first stages of the changing rooms. Disinfection solution dispensers to wash the hands (with and without sterile gloves) should be provided at the last stage of the cleanroom before entering the bioburden controlled environment.
In cleanroom
Disinfection solution dispensers to wash the hands (with sterile gloves) should be provided in several locations inside the bioburden controlled environment. Alcohol based dispensers for sterile gloves and face masks should be provided inside the bioburden controlled environment.
Preparation room
Cleaning and sterilization facilities should be available in the preparation area to the bioburden controlled environment. This should include:
Sonic or precision cleaning.
DHMR facility.
Bagging capability in biobarriers, foils, and ESD for sterilization, and transport.
Storage space
Storage should be provided for sterilized tools and kits.
Monitoring
Back-up systems for cleanroom monitoring should be in place.
Independent of the status of the cleanroom control equipment (e.g., HVAC system), specific cleanroom levels can only be credited if monitored.
Operational guidelines
Surveillance
Continuous video surveillance should be used for all critical areas in bioburden controlled environments during operations. The documented activities should be used to trace back any contamination event detected by one of the regular microbiological assessments. All individuals in the cleanroom should be clearly identifiable by the video surveillance system (e.g., either specific symbol or colour coding of garment).
Communications
Radio communication between personnel inside the bioburden controlled environment and with personnel outside the bioburden controlled environment should be considered. This is especially recommended in laminar flow environment.
Packaging
Multiple bagging should be used whenever possible to facilitate storage on-site and transport through the different cleanroom areas. Unpacking (removal of biobarriers), inspection, cleaning, sterilization, and related activities on and flight hardware should be conducted outside the bioburden controlled environment.
Flight hardware
The flight hardware should have sole occupancy of the bioburden controlled environment in all facilities. If this cannot be guaranteed, measures to prevent cross contamination should be established.
Maintenance
When equipment maintenance has been carried out within the bioburden controlled environment, the area should be cleaned, disinfected or sterilized where appropriate before processing can recommence if the required standards of biological (and particulate) cleanliness have not been maintained during the work.
Fumigation of bioburden controlled environments can be useful for reducing the viable microbiological contamination
Personnel access records
All personnel entering the bioburden controlled environment should be logged.
Task planning
For planning tasks, the planning addresses the fact that it can take experienced personnel 24 times longer to perform mechanical or electrical assembly under bioburden controlled conditions.
Garments
Ready-to-use (e.g., sterile) garment should be available in all required sizes.
Microbiological laboratory
A microbiology laboratory to analyse the assays should be in close proximity (not necessarily at the premise) to the bioburden controlled environment.
Cleanroom bioburden sampling
During the commissioning phase, one surface sample per square meter and per monitoring interval in a bioburden controlled environment should be used. During the routine monitoring phase one surface sample per two square meters and per monitoring interval in a bioburden controlled environment should be used.
The details of the environment and the operations to be carried out can affect the numbers of samples per surface area.
Medical monitoring of personnel
Medical screening
Medical screening for key personnel with access a bioburden controlled environment should start early enough to evaluate the impact on personnel selection. By experience, a certain percentage from the general public is unfit for work in a bioburden controlled environment (1/60-1/200). Medical conditions of concern are chronic bacterial or fungal conditions that cause excessive shedding of skin scale. Smokers release a higher amount of particulates in the period immediately after smoking. Limitations on smoking prior of entering a bioburden controlled environment should be considered.
Illness
Personnel who are ill should not be permitted access to the bioburden controlled environment if the medical condition results in either excessive particle generation, or in the inability to work in the appropriate garment. Provisions should be made for back-up crew during seasonal cycles of potential medical conditions (e.g., flu, allergies).
Bioburden monitoring
Garments
Monitoring should be accomplished by obtaining surface samples of each operators’ gloves on a daily basis. This sampling should be accompanied by an appropriate sampling frequency for other strategically selected locations of the gown.
Intensive working
The responsible unit should establish a more comprehensive monitoring program for operators involved in operations which are especially labour intensive (i.e. those requiring repeated or complex aseptic manipulations).
Unacceptable operator bioburden levels
When operator bioburden levels show an increase trend in bioburden levels an investigation should be conducted promptly. Follow-up actions should include increased sampling, increased observation, retraining, and gowning re-qualification. Where levels do not subsequently return to normal, reassignment of individuals to operations outside the controlled area should be initiated.
Gowning
Gowning Procedure
A detailed gowning procedure should be established and implemented. This should also be part of the training. The clothing and its quality should be appropriate for the process and the cleanliness level of the cleanroom.
Prior to arrival at work
Personnel should preferably bathe or shower the night before in order to allow natural skin oils to be re-established overnight. This practise assists in the reduction of skin particle shedding within the bioburden controlled cleanroom.
Prior to entry into the bioburden controlled facility
Personnel should remove all personal effects (e.g. watches, rings, bracelets, any other jewellery, and mobile phones) and any applied cosmetics. All outer garments should be removed and intermediate garments (e.g. “scrub suits” or other undergarments, beard masks, mob caps, and shoe covers) donned. Hands should be washed with disinfectant or detergent and dried with dispensable towels.
On Entry into final gowning area
Personnel should enter room making full use of appropriate contamination control measures, e.g. air showers, tacky mats, and locate washing and disinfection fluids and materials.
Gloves
Select appropriate size of sterile gloves. When handling gloves avoid contact with any external sterile surface. Do not pick up gloves by fingers of the gloves or outside of glove wrist. Always handle the inner surfaces of the gloves only, i.e.; inner surface of wrist of glove. If any inadvertent handling of the outer surface of the glove occurs during donning, these should be removed and discarded and a new pair selected. Disinfect hands and gloves using solutions provided.
Hood
Select sterile hood of appropriate size. Check packaging to ensure it is still intact and that the items are within the prescribed expiry date. Discard any items that are past the expiry date or where the sterile packaging appears compromized. Open sterile packaging. Handle hood by inner surfaces only. Don hood in one smooth motion, ensuring only inner surfaces of hood make contact with head and shoulders. If any inadvertent contact of head or body with the outer surface of the hood occurs, the hood should be removed and discarded and a new hood selected. Once hood is fitted, check in gowning mirror to ensure hood is correctly positioned over shoulders. Disinfect hands and gloves using solutions provided.
Face-Mask
Remove mask from packaging, taking care to handle by the ties only. Position mask over hood and face, again using only the ties. Once in position, secure the mask with the ties and check the positioning in the gowning mirror. Ensure no skin is visible. Disinfect hands and gloves using solutions provided.
Coverall
Select coverall of appropriate size. Remove coverall from packaging, handling only the inner surfaces. Allow to fall to full length, taking care to avoid contacting the floor and outer surfaces of the coverall with garments currently worn. Unzip if needed, taking care to limit the contact of gloved had to the zip tag only. If possible, unzip from the inside of the garment.
Face coverall away from you, and gather up the arms and extra coverall, touching only the internal surfaces. Step into one leg of the coverall, avoiding contacting the coverall with the floor. Hand-rails to assist in balancing are suggested as an aid to this operation. Repeat operation with other leg, then carefully pull coverall up over shoulders, avoiding contact of any part of the body or undergarments with the outer surface. Carefully tuck hood into coverall. Zip up coverall and secure with snap-studs. Check Coverall and hood are fitted correctly in gowning mirror. Disinfect hands/gloves using solutions provided.
Boots
Select appropriate sterile over-boot size. Check packaging to ensure it is still intact and that the items are within the prescribed expiry date. Discard any items that are past the expiry date or where the sterile packaging appears compromized. Open sterile packaging. As per the procedure for donning gloves, take care to avoid all contact with the sterile outer surfaces of the boot. Handle boots by inner surfaces only. Roll down the boot from the top, then pull boot over one foot and swing leg over boot-bar/bench into clean sector of gowning area. Leave the top of the boot rolled down. Repeat procedure with other boot. If any inadvertent handling of the outer surface of the boot occurs during donning, these should be removed and discarded and a new pair selected. As with donning of gloves, this procedure cannot appropriately performed without some training and practise. Disinfect hands and gloves using solutions provided.
Final fitting of over-boots
Carefully roll tops of boots over legs of coverall to full extent, and secure with ties if applicable. Check entire dress is fitted in accordance with gowning procedure guidelines. Disinfect hands and gloves using solutions provided.
Goggles/Visors
Select a pair of sterilized or sanitized goggles and removed from packaging. Handle goggles by straps/ties only. Position over eyes, ensuring all remaining portions of face are covered. Check fitting in gowning mirror. Disinfect hands and gloves using solutions provided.
Second gloves
Select a pair of sterile gloves of the next size up to the ones used for gowning. Remove from sterile packaging, handling by inner surfaces only. Don gloves over first gloves using same procedure as for first pair, but with the exception of ensuring that the wrists of the second pair extend over the sleeves of the coveralls, thus effecting a particulate seal over the sleeves. Disinfect hands and gloves using solutions provided.
Entering cleanroom
Perform final visual check of all aspects of dress code. Dress should comply with dress code requirements. Proceed into Cleanroom.
Cleanroom cleaning
Cleaning programme
The cleaning program for bioburden controlled environment should include regular cleaning of the environment with disinfectants and with sporicidal disinfectants.
Cleaning procedures
Cleaning procedures should include among others a detailed description of areas to be cleaned, cleaning tools, cleaning solutions and cleaning operations requirements, gowning and safety requirements and the detailed sequence of cleaning operations. Alternating different types of disinfectants on a periodic basis should be used to avoid build-up of resistant microbial population.
Cleaning records
A system for formal scheduling and recording of all routine cleaning operations performed in the biologically controlled cleanroom should be created. This should include a Cleaning Record Sheet which identifies the areas/locations cleaned and the frequency (periodicity) of each cleaning operation (i.e.; whether per shift, daily, weekly, or some other interval).
Reviews
Regular review and reassessment of cleaning efficiency should be performed based on the results of the monitoring program.
Cleaning frequency
Suggested cleaning frequencies and agents are:
All surfaces (including floor) with a synthetic phenolic (contact time 10 minutes) after each shift. Alternate between pH 2 and 11 on a monthly basis.
All surface in direct contact with the product (e.g., flight hardware) should be cleaned with alcohol (e.g., IPA 70 % or ethanol 70 %) (contact time 5 minutes) at least for each shift. 6 % hydrogen peroxide (contact time 15 minutes) can be added to provide sporicidal effect.
Once per month (or before critical operations) the bioburden controlled environment can be disinfected with a dry fogging system using a mixture of paracetic acid and hydrogen peroxide (for sporidical effect) for decontamination of even inaccessible places.
Material compatibility with solutions
All cleaning, disinfection, and sterilization agents used should be compatible with the surfaces to which they are applied. Material compatibilities with cleaning or disinfection solutions should be evaluated before using those agents.
Validation of cleaning/disinfection
In-situ validation of the cleaning and disinfection procedures should be performed during the commissioning phase. If no labelling claims of disinfectant or sterilization efficiency are known, in vitro validation of the disinfectant/sterilization efficiency should be performed.
Recurrent and critical environmental isolates should be used in the in-vitro tests.
Cleaning solutions
Cleaning solutions should be 0,2 µm-filtered to provide for sterilization. Water for the preparation of cleaning solutions should be of or Type I A quality according to ASTM D1193.
Disinfectant solutions
Where disinfectants are used, more than one type should be employed.
Only use disinfectants on clean surfaces. Dust, grease or other debris can render the disinfection process ineffective.
Cleaning sequence
The cleaning sequence should generally move from the cleanest area to the less clean area, ending with the down and de-gown rooms.
Cleaning restrictions
Cleaning should only be conducted during times of no work on flight hardware. Flight hardware should be covered during cleaning.
Vacuum cleaning
A central vacuum system with external exhaust should be used.
HEPA filtered units with internal exhaust should not be used.
Cleanroom working disciplines
Overview
All types and classes of cleanrooms should have appropriate working disciplines in order to achieve and maintain the desired cleanliness levels, and to ensure critical hardware is not contaminated. The following are considered applicable to bioburden controlled cleanrooms.
Access control
Personnel
Only essential personnel should be allowed into the cleanroom. Numbers of personnel within the cleanroom should be kept to the minimum necessary at any one time.
Contamination levels and contamination potential increase as the number of personnel increase.
Untrained Personnel/Visitors
Untrained personnel or casual visitors should not be permitted Only fully trained personnel or trainee personnel under appropriate trained supervision should be permitted access.
Access routing
Personnel should use the designated personnel access route only to the cleanroom, via the changing, antiseptic wash and gowning area. Access via equipment or hardware transfer ante-rooms (e.g. those routes used for large MGSE etc.) should not be used as an alternative means of access into the bioburden controlled area.
Doors
Doors should not be left or wedged open. Doors to another area should not be opened whilst the door to the preceding (less clean) area is still open.
Ideally, this should be rendered impossible by electronic interlocking.
Materials:
Contaminating materials should not be taken into the cleanroom. If in doubt as to the suitability of ingress or use of any given material, the relevant Planetary Protection Responsible Engineer/Manager should be consulted. This extends to all cleanroom consumables (wipes, solvents, detergents, swabs etc.) which can be employed. A list of approved cleanroom consumables should be produced. Care should also be exercised in the uses of uncontrolled items by cleaning staff. All materials and equipments used for surface cleaning (mops, wipes, tacky rollers, detergents/surfactants, vacuum cleaners) should be dedicated and approved items.
The following lists some examples of common contaminating materials which may be introduced by personnel working in the area, but is not exhaustive:
Paper (e.g. documents, packaging, tissues, books, newspapers).
Food, drinks, confectionary.
Personal jewellery.
CD Players, MP3 players, radios, mobile phones, electronic organizers.
Abrasives or powders.
Aerosols (e.g. perfumes, deodorants).
Organic or natural materials e.g. wood, rubber, leather, cotton.
Other personal effects such as tobacco products, purses, wallets, spectacle cases, car or door keys, currency, etc.
Unauthorized writing implements (certain pens, pencils, marker pens).
Audits
Periodic audits of the cleanroom for presence of non-compliant materials should be performed.
Working practises
Locomotion
Movements within the cleanroom should be slow and controlled. Erratic and rapid movements generate more particulate contamination and disturb the optimum airflow.
Housekeeping
Good housekeeping is important. Areas where items are closely positioned or cluttered act as contamination traps and the result is stagnant air pockets which are not removed by the normal unimpeded airflow.
Positioning of personnel
Personnel should be positioned down-stream of the air flow past the product. Leaning over the product increases the risk of particulate, organic and microbial contamination. Talking whilst facing or leaning over the hardware should not be permitted, even when wearing full face masks.
Personnel should not cough or sneeze whilst still facing critical hardware. If coughing or sneezing is unavoidable, the face should be turned away. Masks should be replaced after any such incident.
Planning
Before carrying out any operation in the cleanroom, the activity(s) should be thought through and pre-planned in order to minimize contamination potential.
Hand-carried objects
When hand-carrying items in the cleanroom, these should not be held tight against the cleanroom garments, e.g. pulled into the stomach or chest, or under one arm. The outside of the garments are not 100 % clean, and friction between the object being carried and the garment generates particles and fibres. These and any associated bacteria can thus be transferred onto hardware or objects.
Handling of objects/surfaces
Wherever possible, objects should not be handled directly – e.g. sterilized tweezers or forceps can be used instead of gloved hands, which have a greater contamination potential than sterilized instruments. Personnel should avoid touching cleanroom surfaces unnecessarily. The recommended posture for the hands at rest is clasped in front of the body. Periodic disinfectant washing of the gloves as per that employed during the gowning procedure should be performed, particularly prior to critical handling operations, after contact with known contaminants, or after prolonged handling events.
Contact with non-flight hardware
Contact with non-flight items such as cabling; wiring or vacuum hoses should be avoided Lay-flat polymer tubing with appropriate ESD control should be used to encapsulate cable bundles.
Cleanroom wipes/swabs
Wipes or swabs should be used only once and then discarded.
Bibliography
|
ECSS-S-ST-00
|
ECSS system – Description, implementation and general requirements
|
|
ISO 14698 part 1
|
Cleanrooms and associated controlled environments - Biocontamination control - Part 1: General principles and methods
|
|
ISO 14698 part 2
|
Cleanrooms and associated controlled environments - Biocontamination control - Part 2: Evaluation and interpretation of biocontamination data
|
|
IEST-RP-CC023.2
|
Microorganisms in Cleanrooms
|
|
ASTM D1193
|
Standard specification for reagent water
|